Chapter 7 Chemistry Test
Chapter 7 Chemistry Test - Negative ions negative ions are called? 48 questions | by konlee | updated: Valence electrons what is formed when atoms gain electrons? N2h4(l) + h2(g) → 2nh3(g) reaction 2: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Web octet metals are reactive because they lose what easily? Mar 21, 2022 | attempts: 7.6 molecular structure and polarity; Web 7.5 strengths of ionic and covalent bonds; Click the card to flip 👆 1 / 11 flashcards learn test.
Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Web 7.5 strengths of ionic and covalent bonds; Web chapter 7 chem: Click the card to flip 👆 1 / 11 flashcards learn test. Negative ions negative ions are called? 48 questions | by konlee | updated: Mar 21, 2022 | attempts: Web octet metals are reactive because they lose what easily? 7.6 molecular structure and polarity; Valence electrons what is formed when atoms gain electrons?
Negative ions negative ions are called? Web 7.5 strengths of ionic and covalent bonds; 7.6 molecular structure and polarity; Web chapter 7 chem: Click the card to flip 👆 1 / 11 flashcards learn test. Mar 21, 2022 | attempts: Valence electrons what is formed when atoms gain electrons? 48 questions | by konlee | updated: N2h4(l) + h2(g) → 2nh3(g) reaction 2: Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end?
S block elements Chapter 7 Chemistry Class 11 MHTCET 2020 2021
Web chapter 7 chem: Click the card to flip 👆 1 / 11 flashcards learn test. 48 questions | by konlee | updated: Valence electrons what is formed when atoms gain electrons? N2h4(l) + h2(g) → 2nh3(g) reaction 2:
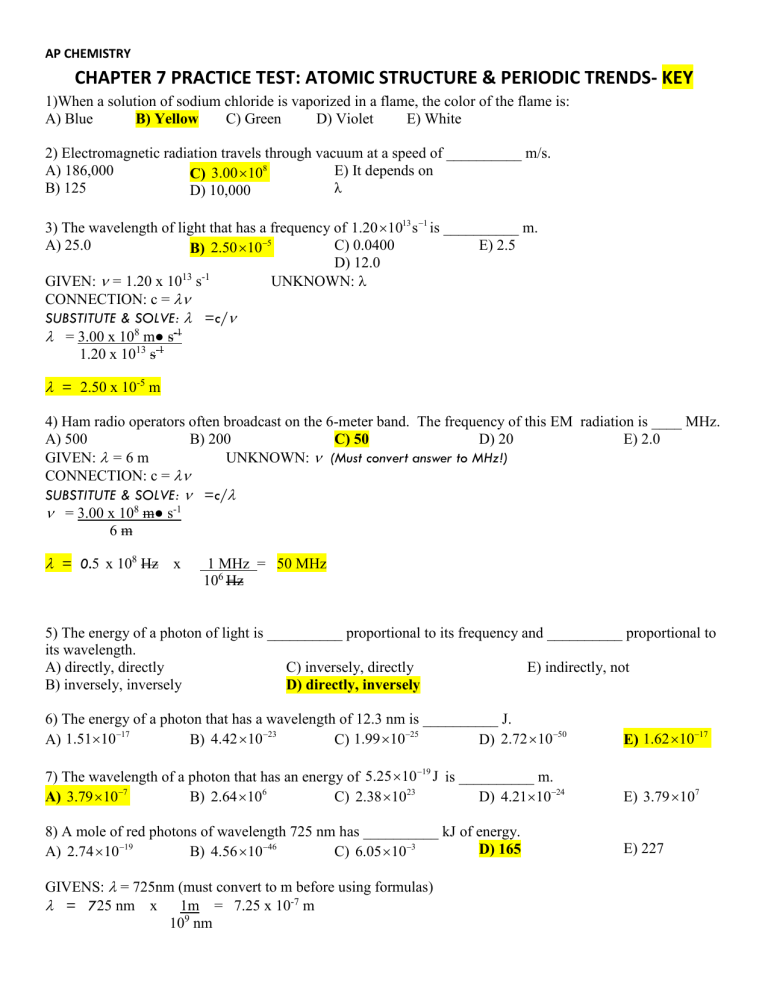
AP CHEMISTRY CHAPTER 7 PRACTICE TEST ATOMIC
Web 7.5 strengths of ionic and covalent bonds; Mar 21, 2022 | attempts: Valence electrons what is formed when atoms gain electrons? Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Negative ions negative ions are called?
Chemistry Chapter 7 2nd Year
Valence electrons what is formed when atoms gain electrons? Web 7.5 strengths of ionic and covalent bonds; Mar 21, 2022 | attempts: Web octet metals are reactive because they lose what easily? N2h4(l) + h2(g) → 2nh3(g) reaction 2:
NCERT Exemplar Class 12 Chemistry Solutions Chapter 7 The P Block
N2h4(l) + h2(g) → 2nh3(g) reaction 2: Web octet metals are reactive because they lose what easily? Click the card to flip 👆 1 / 11 flashcards learn test. 48 questions | by konlee | updated: Web chapter 7 chem:
Chapter 7 Chemistry YouTube
Negative ions negative ions are called? Web octet metals are reactive because they lose what easily? Valence electrons what is formed when atoms gain electrons? Web 7.5 strengths of ionic and covalent bonds; Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end?
Chapter 7 Chemistry Test Answers Study Finder
N2h4(l) + h2(g) → 2nh3(g) reaction 2: Web 7.5 strengths of ionic and covalent bonds; Web octet metals are reactive because they lose what easily? Valence electrons what is formed when atoms gain electrons? 48 questions | by konlee | updated:
Chemistry Chapter 7 Worksheet Answers —
Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Web chapter 7 chem: Web octet metals are reactive because they lose what easily? Valence electrons what is formed when atoms gain electrons? N2h4(l) + h2(g) → 2nh3(g) reaction 2:
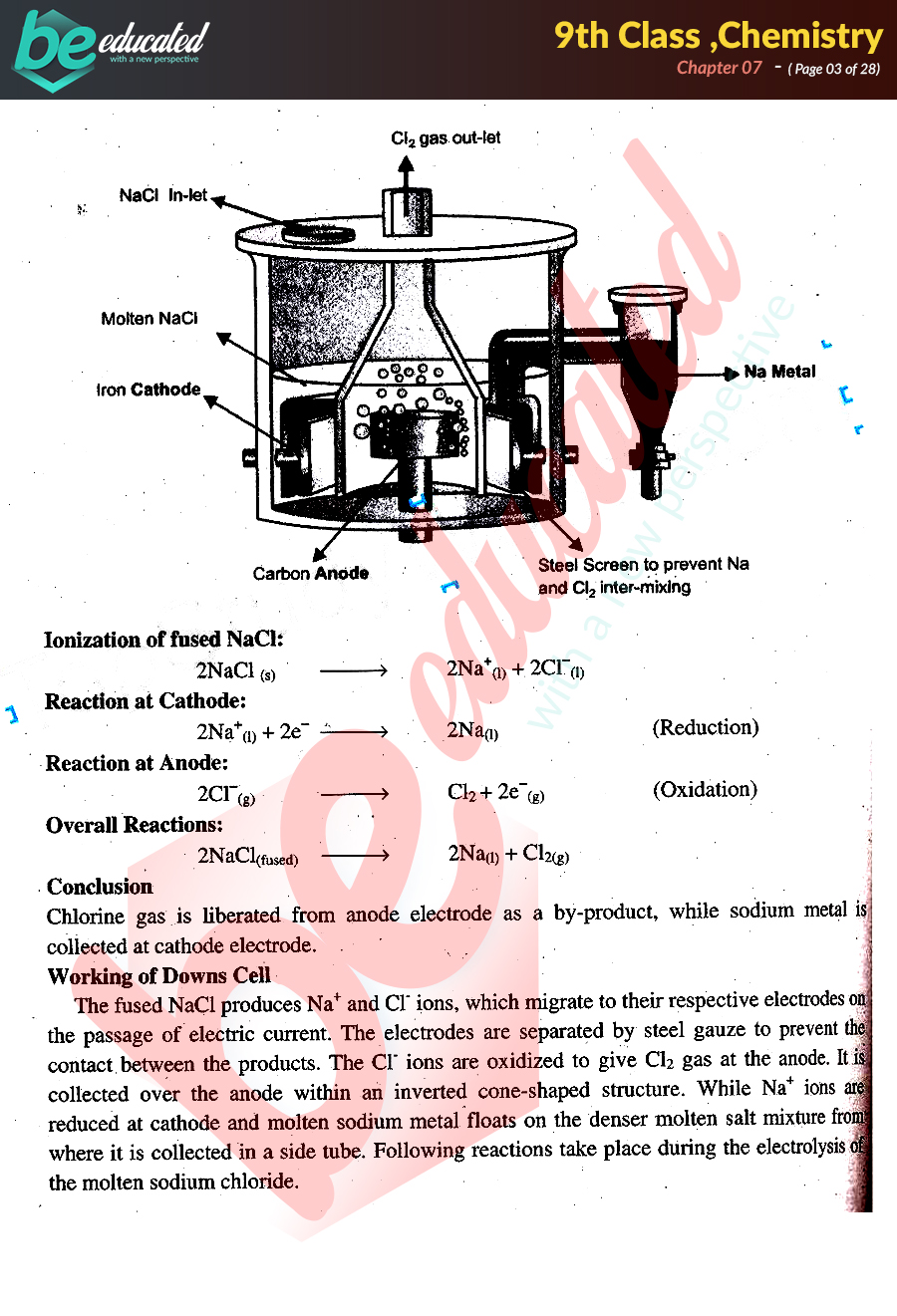
Chapter 7 Chemistry 9th Class Notes Matric Part 1 Notes
N2h4(l) + h2(g) → 2nh3(g) reaction 2: Web octet metals are reactive because they lose what easily? Web 7.5 strengths of ionic and covalent bonds; Web chapter 7 chem: 48 questions | by konlee | updated:
Chapter 7 Test Review
Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end? Valence electrons what is formed when atoms gain electrons? Mar 21, 2022 | attempts: N2h4(l) + h2(g) → 2nh3(g) reaction 2: 48 questions | by konlee | updated:
Web Chapter 7 Chem:
Negative ions negative ions are called? N2h4(l) + h2(g) → 2nh3(g) reaction 2: Valence electrons what is formed when atoms gain electrons? Click the card to flip 👆 1 / 11 flashcards learn test.
7.6 Molecular Structure And Polarity;
Mar 21, 2022 | attempts: 48 questions | by konlee | updated: Web octet metals are reactive because they lose what easily? Web chemistry chapter 7 test sigma click the card to flip 👆 what type of bond forms as a result of orbitals overlapping end to end?